Making a Difference, Patient by Patient
Specialty pharmaceuticals with a personal touch—improving lives by licensing, developing, and delivering medicines for patients with rare and challenging conditions.
Patients are Key to Everything that We Do at US Worldmeds

Vision
We will remain a sustainable, privately held specialty pharmaceutical company.

Mission
We will develop, license, and market meaningful and accessible healthcare products that improve lives and result in a thriving community of patients, employees, and shareholders.

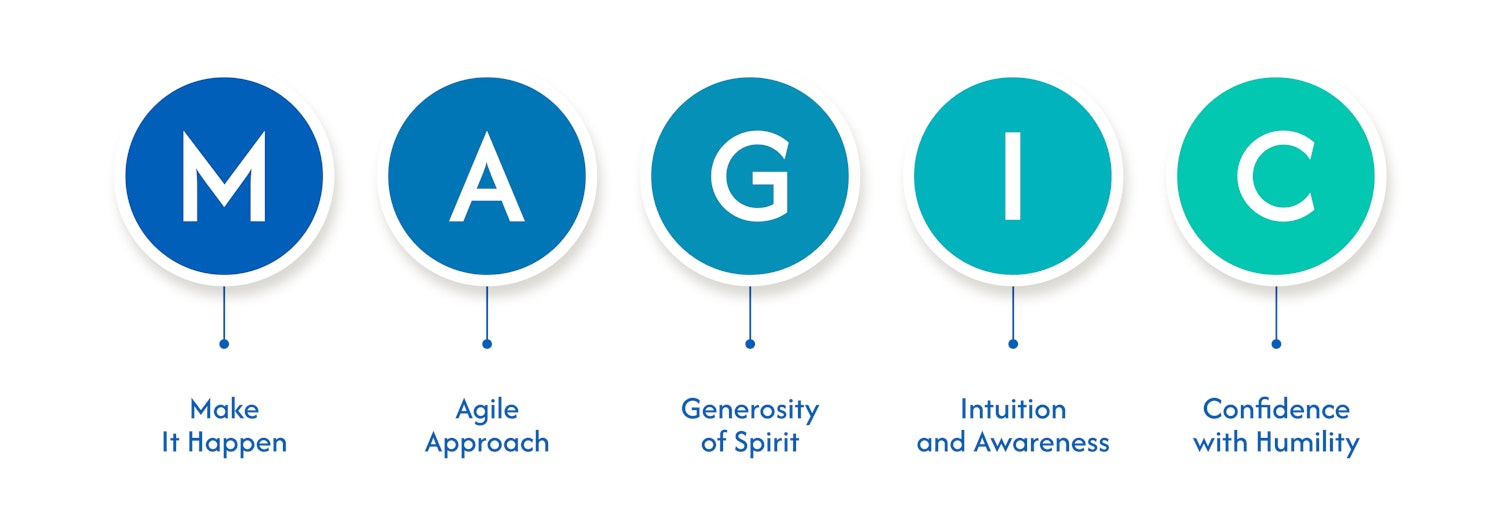
Values
Corporate Giving
US WorldMeds is committed to improving the lives of patients with challenging conditions and unmet medical needs. In alignment with this mission and the company’s Generosity of Spirit core value, USWM makes available philanthropic funding opportunities for qualified not-for-profit organizations that further the study, treatment, awareness, or patient outcomes of diseases in our areas of focus.
USWM ensures corporate giving is conducted in compliance with industry codes, relevant laws and regulations, and USWM’s policies and procedures. A recipient’s use, purchase, or prescription of USWM products or a recipient’s ability to influence the use, purchase, or prescription of USWM products will not be considered when deciding charitable giving. Eligible applicants will be tax-exempt organizations under Section 501(c)-(3) or 501(c)-(6) of the U.S. Internal Revenue Code.
USWM does not offer funding for government agencies, political purposes, religious purposes, or for any other purpose that would pose a conflict of interest or contradict USWM’s mission.
Requests for unsolicited charitable contributions will be received at corpgiving@usworldmeds.com.

